"FDA Approves Darzalex Combo Therapy For Multiple Myeloma Patients" - Carolina Henriques
The US Federal Drug Administration has approved a combination of the immunotherapies Darzalex (daratumumab), Pomalyst (pomalidomide) and dexamethasone as a multiple myeloma treatment. The combo offers a new option for patients who fail to respond to two lines of therapy that include Revlimib and a proteasome inhibitor like Velcade. "Despite tremendous progress, most patients with multiple myeloma continually relapse or become resistant to available therapies, such as proteasome inhibitors and immunomodulatory agents," said Ajai Chari, MD, associate professor of hematology and medical oncology at the Icahn School of Medicine at Mount Sinai. Therefore, these patients continue to need new options. With today's approval of Darzalex, "we now have a promising new combination therapy that in clinical trials demonstrated pronounced clinical benefit for patients who have relapsed on two of the most widely used treatments" Dr. Chari adds.
- Ajai Chari, MD, Associate Professor, Medicine, Hematology, Medical Oncology, Icahn School of Medicine at Mount Sinai
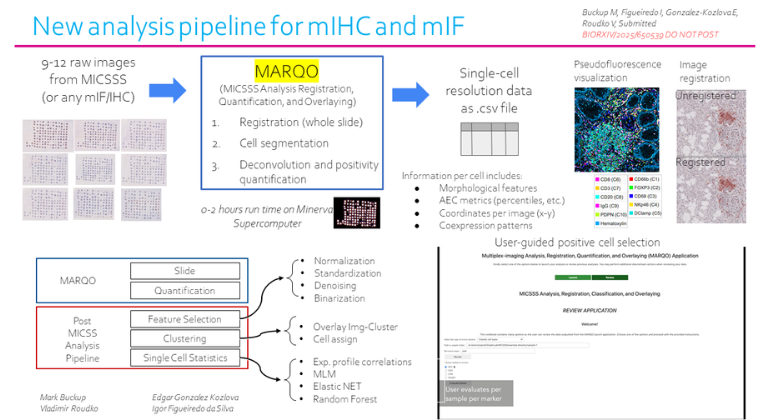
Mount Sinai Scientists Create AI-Powered Tool to Improve Cancer Tissue Analysis
Aug 25, 2025 View All Press Releases
Combination Therapy Improves Outcomes for Advanced Triple-Negative Breast Cancer
Aug 21, 2025 View All Press Releases
The Mount Sinai Hospital Ranked Among Top in the Nation by U.S. News & World Report®
Jul 29, 2025 View All Press Releases
Mount Sinai Researchers Engineer Rare Immune Cells to Create Powerful New Cancer Vaccine
Jul 21, 2025 View All Press Releases