Mount Sinai Researchers Uncover the Biology and Treatment Behind a Rare Autoinflammatory Disease
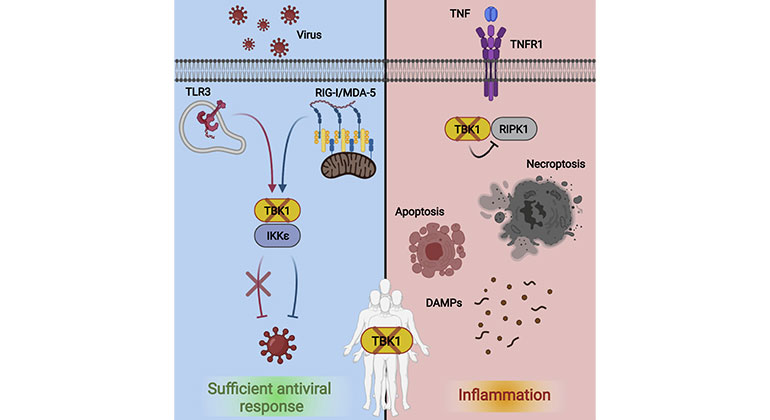
The absence of a protein that activates the body’s antiviral defenses can cause a rare rheumatoid-like autoinflammatory condition that is treatable with an FDA-approved class of drugs known as TNF (tumor necrosis factor) inhibitors, a global research team led by Mount Sinai has found.
The condition, which is now termed TBK1 deficiency, was previously known to scientists but its name, cause and treatment were identified for the first time in the study published August 6 in Cell.
The scientists reported that the absence of the protein, known as TBK1 (TANK-binding kinase 1), renders cells vulnerable to a jarring form of programmed cell death in response to TNF, but that this genetic defect can be effectively and quickly addressed by therapeutic agents that block the source of the inflammation.
Homozygous mutations in TBK1, which occur when copies of the aberrant gene encoding the protein are passed on by both parents, are extremely rare. Based on past studies in mouse models and human cell cultures, researchers assumed that these mutations would leave individuals susceptible to a wide range of viral infections. They found instead that none of the four people in the cohort they studied, ages 7 to 32, showed signs of inadequate antiviral immunity. Rather, they all suffered from a systemic autoinflammatory condition that resulted from a dysregulated response to TNF, an important protein involved in controlling inflammation and cell death.
“TBK1 signaling activates the body’s antiviral mechanisms to fight off infection and block different stages of viral replication, as well as control TNF-mediated inflammation,” says lead author Justin Taft, PhD, an investigator in the Department of Microbiology, the Precision Immunology Institute, and the Center for Inborn Errors of Immunity at the Icahn School of Medicine at Mount Sinai. “But if a mutation prevents expression of the TBK1 gene or disrupts its function, then cells become overly sensitive to TNF. And that can trigger a disproportionate amount of cell death, which sets off a violent cascade of debris from dying cells that inflames surrounding tissue and fuels the inflammation.”
By treating TBK1-deficient individuals with anti-TNF therapeutics, the Mount Sinai-led team confirmed its suspicions about the underlying biology of the genetically driven condition. “We have essentially defined a new disease and its associated mechanisms of autoinflammation, which previously were managed with steroid treatments, non-steroidal anti-inflammatory drugs, or other non-specific therapeutics clinicians deemed worth trying,” says Dusan Bogunovic, PhD, Director of the Center for Inborn Errors of Immunity; Associate Professor of Oncological Sciences, Microbiology, and Pediatrics; member of the Precision Immunology Institute and The Mindich Child Health and Development Institute; and senior author of the study. “We were able to target the condition directly and effectively with TNF inhibitors once we knew the causative factors of the inflammation. And the clinical improvement was quick and substantial.”
For Dr. Taft, the work of the global team of researchers underscores the power of the increasingly vital field of personalized medicine. “We started with four individuals who were known to have a homozygous TBK1 mutation, and did extensive lab work to determine how the defect could induce this autoinflammatory reaction,” says Dr. Taft. “And from the genetics we uncovered not only a plausible mechanism of the disease and new information around TBK1 biology, but identified a disease-specific treatment that significantly improves the autoinflammatory condition.”
In addition to Mount Sinai, the collaborative research effort included Erasmus University Medical Center in the Netherlands, Mayo Clinic, the National Human Genomic Research Institute, Imperial College London, SRCC Children’s Hospital and Jaslok and Breach Candy Hospitals in Mumbai, India, and Hacettepe University in Ankara, Turkey.
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, employing 48,000 people across its hospitals and more than 400 outpatient practices, as well as more than 600 research and clinical labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time—discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 9,000 primary and specialty care physicians and 11 free-standing joint-venture centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida. Hospitals within the System are consistently ranked by Newsweek’s® “The World’s Best Smart Hospitals, Best in State Hospitals, World Best Hospitals and Best Specialty Hospitals” and by U.S. News & World Report's® “Best Hospitals” and “Best Children’s Hospitals.” The Mount Sinai Hospital is on the U.S. News & World Report® “Best Hospitals” Honor Roll for 2024-2025.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Twitter and YouTube.