Mount Sinai Researchers Move Closer to a Cure for Diabetes
New research confirms a novel route for human beta cell regeneration
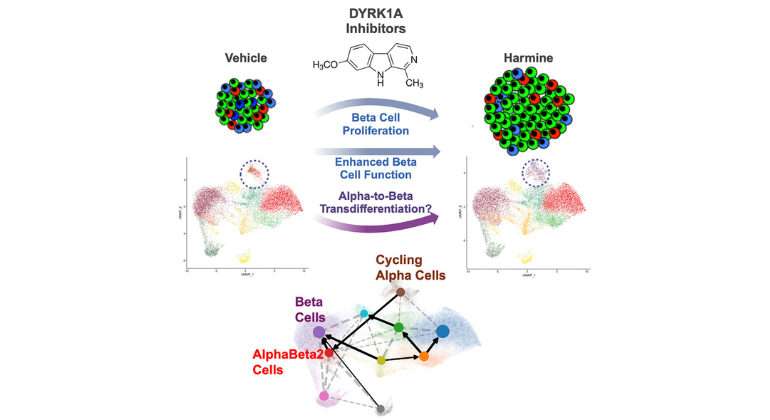
Graphical abstract of the work.
Diabetes researchers and bioinformaticians from the Icahn School of Medicine at Mount Sinai have developed a new understanding of how human beta cell regenerative drugs work. These drugs, developed at Mount Sinai, may hold promise for more than 500 million people with diabetes in the world. The results of this study were published this month in Cell Reports Medicine [DOI: 10.1016/j.xcrm.2024.101832].
Diabetes develops when cells in the pancreas known as beta cells become unable to produce insulin, a hormone that is essential to regulating blood sugar levels. While great progress has been made toward discovering a durable therapy, none are scalable therapeutic options for millions of diabetics across the globe.
For more than 15 years, researchers at the Icahn School of Medicine at Mount Sinai have worked tirelessly to find a solution to cure diabetes by identifying a drug that could make human beta cells regenerate.
In 2015, Mount Sinai researchers discovered the first such drug, called harmine. Harmine is a member of a class of drugs called DYRK1A inhibitors. In 2019 and 2020, the researchers reported that DYRK1A inhibitors can synergize with TGF-beta signaling as well as GLP-1 receptor agonist (GLP-1RA) drugs such as semaglutide (e.g., Ozempic) and exenatide (Byetta) to induce more robust levels of human beta cell regeneration. Finally, in July 2024, they showed that harmine alone increases human beta cell mass by 300 percent, and if a GLP-1RA is added, by 700 percent.
A key question has been how harmine causes beta cells to regenerate. In the newest study, the research team reports that the new, regenerated beta cells may be coming from an unexpected source: a second pancreatic cell type called alpha cells. Since alpha cells are abundant in people with type 1 and type 2 diabetes, they may be able to serve as a source for new beta cells in both common types of diabetes.
“This is an exciting finding that shows harmine-family drugs may be able to induce lineage conversion in human pancreatic islets,” says Esra Karakose, PhD, Assistant Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at the Icahn School of Medicine at Mount Sinai and corresponding author of the study. “It may mean that people with all forms of diabetes have a large potential ‘reservoir’ for future beta cells, just waiting to be activated by drugs like harmine.”
“It has been remarkable and rewarding to watch this multi-group story unfold over the past 15 years,” added Andrew F. Stewart, MD, Irene and Dr. Arthur M. Fishberg Professor of Medicine at the Icahn School of Medicine at Mount Sinai and Director of the Mount Sinai Diabetes, Obesity, and Metabolism Institute. He and Peng Wang, PhD, Professor of Medicine (Endocrinology, Diabetes and Bone Disease) at the Icahn School of Medicine at Mount Sinai, conceived of and performed the initial high-throughput drug screen that led to the discovery of harmine, described in Nature Medicine in 2015.
“A simple pill, perhaps together with a GLP1RA like semaglutide, is affordable and scalable to the millions of people with diabetes,” said Dr. Stewart.
The work represents a combined effort by the Icahn School of School of Medicine at Mount Sinai team: Dan Hasson, PhD, Associate Professor of Oncological Sciences, and Dermatology, and Director of the Bioinformatics and Next-Generation Sequencing (BiNGS) Core; Robert Sebra, PhD, Professor of Genetics and Genomic Sciences, Director of the Center for Advanced Genomic Technology, and Director of Technology Development for the Genomics Core Facility; Robert J. DeVita, PhD, Professor of Pharmacological Sciences and Director of the Medicinal Chemistry Core of the Drug Discovery Institute; and long-term collaborator Adolfo Garcia-Ocana, PhD, formerly a professor at the Icahn School who is now the Ruth B. and Robert K. Lanman Chair in Gene Regulation and Drug Discovery Research and chair of the Department of Molecular and Cellular Endocrinology at City of Hope. The Mount Sinai team is moving these studies to human trials.
The work has been supported for decades with funding from the National Institutes of Health, the National Institute of Diabetes Digestive and Kidney Disease, By BreakthroughT1D, formerly JDRF, as well as through additional generous philanthropic gifts.
About the Icahn School of Medicine at Mount Sinai
The Icahn School of Medicine at Mount Sinai is internationally renowned for its outstanding research, educational, and clinical care programs. It is the sole academic partner for the eight- member hospitals* of the Mount Sinai Health System, one of the largest academic health systems in the United States, providing care to New York City’s large and diverse patient population.
The Icahn School of Medicine at Mount Sinai offers highly competitive MD, PhD, MD-PhD, and master’s degree programs, with enrollment of more than 1,200 students. It has the largest graduate medical education program in the country, with more than 2,600 clinical residents and fellows training throughout the Health System. Its Graduate School of Biomedical Sciences offers 13 degree-granting programs, conducts innovative basic and translational research, and trains more than 500 postdoctoral research fellows.
Ranked 11th nationwide in National Institutes of Health (NIH) funding, the Icahn School of Medicine at Mount Sinai is among the 99th percentile in research dollars per investigator according to the Association of American Medical Colleges. More than 4,500 scientists, educators, and clinicians work within and across dozens of academic departments and multidisciplinary institutes with an emphasis on translational research and therapeutics. Through Mount Sinai Innovation Partners (MSIP), the Health System facilitates the real-world application and commercialization of medical breakthroughs made at Mount Sinai.
-------------------------------------------------------
* Mount Sinai Health System member hospitals: The Mount Sinai Hospital; Mount Sinai Beth Israel; Mount Sinai Brooklyn; Mount Sinai Morningside; Mount Sinai Queens; Mount Sinai South Nassau; Mount Sinai West; and New York Eye and Ear Infirmary of Mount Sinai.
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, with 48,000 employees working across seven hospitals, more than 400 outpatient practices, more than 600 research and clinical labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time—discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 9,000 primary and specialty care physicians and 10 free-standing joint-venture centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida. Hospitals within the System are consistently ranked by Newsweek’s® “The World’s Best Smart Hospitals, Best in State Hospitals, World Best Hospitals and Best Specialty Hospitals” and by U.S. News & World Report's® “Best Hospitals” and “Best Children’s Hospitals.” The Mount Sinai Hospital is on the U.S. News & World Report® “Best Hospitals” Honor Roll for 2025-2026.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Instagram, LinkedIn, X, and YouTube.