Mount Sinai Is First in the Nation to Perform Deep Brain Stimulation Implant as Part of Clinical Trial for Depression
Case marks the beginning of a multi-site pivotal trial to evaluate Abbott’s system for management of treatment-resistant depression

A team of experts from the Icahn School of Medicine at Mount Sinai has become the first in the United States to perform a deep brain stimulation (DBS) implantation procedure as part of a multi-site, randomized controlled trial to investigate the use of healthcare company Abbott’s DBS systems for treatment-resistant depression.
Under the leadership of Brian H. Kopell, MD, Professor of Neurosurgery, Neurology, Psychiatry, and Neuroscience at the Icahn School of Medicine, Director of the Center for Neuromodulation at Mount Sinai West, and national neurosurgical Principal Investigator of the trial, known as TRANSCEND, the first study participant underwent a successful DBS procedure and will be followed within the study over the next three years.
As many as 2.8 million Americans are diagnosed each year with treatment-resistant depression, a silent, yet debilitating condition.¹ A form of major depressive disorder, treatment-resistant depression is diagnosed following the failure of multiple treatment avenues. For individuals who are living with treatment-resistant depression, their chances of finding relief from their symptoms diminish each time a treatment fails—following the fourth failed treatment, as many as 83 percent of patients will relapse into their condition.
“A schism between what is considered ‘psychiatric’ and what is ‘neurological’ exists currently in the lay community and even amongst many clinicians. Advances in neuroscience, however, have made it clear that these ‘psychiatric diseases’ are indeed similar to other neurological conditions—we can see identifiable structural and functional changes in the brain,” said Dr. Kopell. “Therefore, it’s not surprising that deep brain stimulation research thus far has demonstrated promise for people with treatment-resistant depression. We are thrilled to kick off Abbott’s TRANSCEND trial here at Mount Sinai with this first patient case and are eager to help gather further evidence about the impact this experimental treatment could have for patients with treatment-resistant depression.”
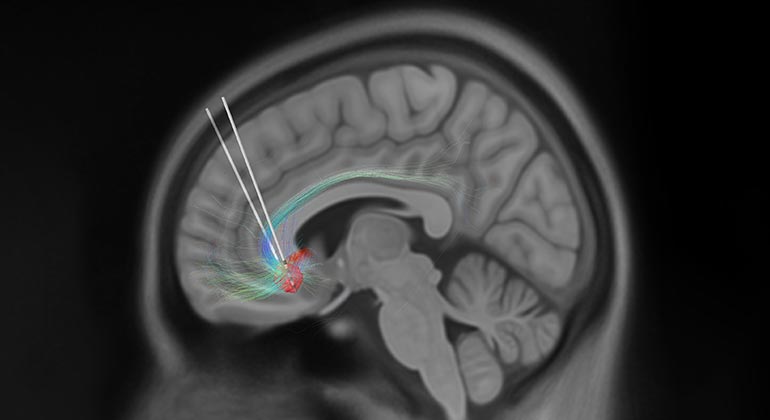
Deep brain stimulation is, in essence, a pacemaker for the brain. During a DBS procedure, the surgeon threads a small wire with an electrode at the end—known as a lead—through a small opening in the skull until the tip reaches a specifically targeted spot deep in the brain, informed by preoperative advanced imaging techniques. The surgeon then connects the wire and electrode to a wristwatch-sized neurostimulator device implanted just under the skin, near the collarbone. This enables the device to send an electrical current, when necessary, to disrupt pathological electrical activity in the brain to help alleviate symptoms. DBS for treatment-resistant depression has not been approved by the U.S Food and Drug Administration (FDA) and the safety and effectiveness of DBS for treatment-resistant depression has not been established. However, DBS is currently approved by the FDA to treat the symptoms of neurological disorders like Parkinson’s disease and essential tremor.
The science behind the potential of deep brain stimulation for treatment-resistant depression has been pioneered by Helen S. Mayberg, MD, Founding Director of the Nash Family Center for Advanced Circuit Therapeutics at Mount Sinai, who is renowned for her study of brain circuits and deep brain stimulation research over the past 20 years. She is credited with identifying the subcallosal cingulate as a signaling hub for depression in the brain and discovering that modulation of this brain area can help alleviate depression symptoms. Previous research, much of it pioneered by Dr. Mayberg through independent, single-arm, open-label, grant-funded studies at several leading academic research institutions and for the past seven years at Mount Sinai, has shown sustained improvement in symptoms of depression.
In September 2022, Abbott received Breakthrough Device designation from the U.S. Food and Drug Administration to investigate DBS for treatment-resistant depression. Mount Sinai is one of 25 clinical trial sites in the United States for the TRANSCEND trial and is expected to recruit five to ten treatment-resistant depression patients to participate in this clinical trial of subcallosal cingulate cortex DBS for depression. Participants will be randomly assigned to either the treatment arm or control arm of the trial. All will be implanted with the Abbott DBS device; those in the treatment arm will then have the system turned on, while those in the control arm will not. Once a participant has completed 12 months in the study, all participants, regardless of which arm they were originally assigned to, will have their DBS system turned on and followed for two years. Martijn Figee, MD, PhD, a psychiatrist at the Nash Family Center for Advanced Circuit Therapeutics and Director of the Mount Sinai Interventional Psychiatry Program, is Principal Investigator of the Mount Sinai site.
“The TRANSCEND trial is an exciting and critical next step in the process of uncovering the potential of deep brain stimulation for patients with treatment-resistant depression,” said Joshua B. Bederson, MD, the Leonard I. Malis, MD / Corinne and Joseph Graber Professor of Neurosurgery and Chair of the Department of Neurosurgery at Mount Sinai Health System. “It is fitting that Mount Sinai has become the first center in the United States to perform a successful implant as part of Abbott’s TRANSCEND trial, as much of the study of DBS for depression has been carefully and methodically championed by members of the Mount Sinai team, who remained steadfast in their work over several decades to get us to where we are today.”
¹Rush AJ, Trivedi, MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi:10.1176/ajp.2006.163.11.1905
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, with 48,000 employees working across seven hospitals, more than 400 outpatient practices, more than 600 research and clinical labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time—discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 9,000 primary and specialty care physicians and 11 free-standing joint-venture centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida. Hospitals within the System are consistently ranked by Newsweek’s® “The World’s Best Smart Hospitals, Best in State Hospitals, World Best Hospitals and Best Specialty Hospitals” and by U.S. News & World Report's® “Best Hospitals” and “Best Children’s Hospitals.” The Mount Sinai Hospital is on the U.S. News & World Report® “Best Hospitals” Honor Roll for 2024-2025.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Instagram, LinkedIn, X, and YouTube.
Artificial Intelligence Platform Screens for Acute Neurological Illnesses at Mount Sinai
Aug 13, 2018 View All Press Releases