
Mount Sinai Heart
Mount Sinai Heart is celebrated internationally as a world leader in all facets of cardiology care, cardiac surgery, and advanced research.
Mount Sinai Heart
Our team of award-winning physicians has invigorated the science of cardiovascular medicine, pioneering treatments for arrhythmias, coronary artery disease, heart failure, valvular disease, and vascular disease.
This specialty report highlights:
- Mount Sinai’s top cardiologist, who co-chairs a national panel on the future of global health
- The Fuster-BEWAT score, which is found to predict subclinical atherosclerosis without lab tests
- The ReChord trial, which is advancing minimally invasive mitral valve repair
- A study that reveals a direct link between surgeon experience and outcomes for mitral valve operations
- Two physicians at Mount Sinai’s Cardiac Catheterization Lab who received a top safety rating
- The Bifurcaid app, a new teaching tool for a challenging procedure
- A clinical trial testing a therapy for patients with ventricular arrhythmias that resist standard treatment
- The STRIVE project, which traces the “heart-brain” mechanisms linking stress and cardiovascular risk
- Remote monitoring that improves readmission rates for patients with congestive heart failure
- Gene therapy that improved heart function in pigs and is set for human trial
- A study that focuses on outcomes for women and minorities who receive PCI with drug-eluting stents
Mount Sinai’s Top Cardiologist Co-Chairs National Panel on the Future of Global Health
Global health investment by the United States has long focused on the detection and treatment of infectious diseases such as tuberculosis and HIV/AIDS, with significant results. A special committee of the National Academies of Sciences, Engineering, and Medicine found that while these efforts should be maintained, there is a pressing need to meet the challenge of cardiovascular disease (CVD) and other noncommunicable diseases.
Fourteen steps to strengthen global-health programs were recommended in a report by the Committee on Global Health and the Future of the United States, which was co-chaired by Valentin Fuster, MD, PhD, Director of Mount Sinai Heart and Physician-in-Chief of The Mount Sinai Hospital.

The report, which was featured in an editorial in The New England Journal of Medicine in September 2017, said that because of improved sanitation and prevention efforts, the burden of disease is shifting from infectious diseases to noncommunicable diseases.
“Chronic illnesses like heart disease and cancer continue to be a worldwide problem,” Dr. Fuster says. Cardiovascular disease was responsible for 18 million deaths in 2015, with the global cost expected to reach more than $1 trillion by 2030 in terms of treatment and loss of productivity. “The cost is huge, and we are not responding,” he says.
The 360-page report, Global Health and the Future Role of the United States, made evidence-based recommendations in four priority areas:
- Ensuring global health security against infectious-disease pandemics
- Addressing communicable threats, like HIV/AIDS, tuberculosis, and malaria
- Investing in women’s and children’s health
- Promoting cardiovascular health and preventing cancer.
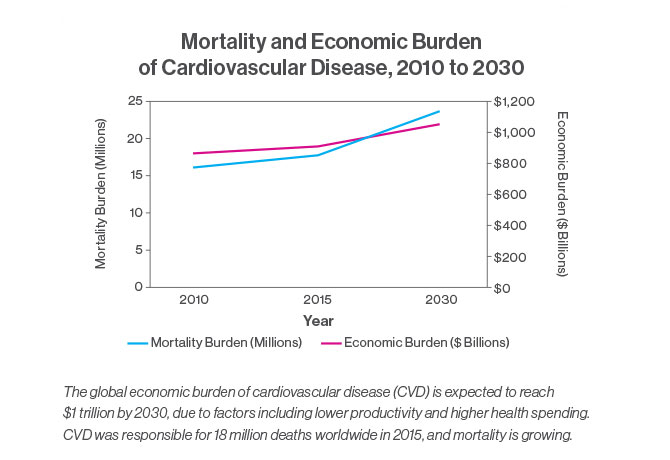
The report’s recommendations on cardiovascular disease were the focus of a December 2017 article in the Journal of the American College of Cardiology. The article noted that mortality due to CVD has been growing around the world, rising 12.5 percent between 2000 and 2015. And the increase was largely attributed to lower- to middle-income countries, where 80 percent of all deaths related to cardiovascular disease occur.
The report cited “best buy” interventions for noncommunicable diseases that would cost $120 billion over 15 years but would drive a 10 percent decrease in CVD-attributable mortality and produce a $377 billion projected economic benefit due to increased productivity and lower health care costs. The interventions called for targeting risk factors with population-level measures, such as tax increases on tobacco and alcohol, and point-of-service measures, such as counseling and drug therapy for people with a high risk of heart attacks.
The report also recommended expanded screening for high blood pressure and other CVD risk factors, which could often be integrated with services already offered through U.S. global-health programs. Screening services could also be integrated with communicable-disease programs. For example, the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) has formed a public-private partnership with AstraZeneca called Healthy Heart Africa, which integrates HIV infection-reduction programs with hypertension screening targeting older men.
To have the greatest effect in its priority areas, the Committee identified three “areas for action” to maximize the return on investments, achieve better health outcomes, and use funding more effectively. They were: accelerating the development of medical products and digital health tools; employing more flexible financing to encourage new partners and funding in global health; and maintaining the status of the United States as a leader in global health.
Investing in global health contributes significantly to economic prosperity and stability and creates more reliable and durable partners in the world, the report said, noting that 11 of the top 15 trading partners of the United States are former recipients of foreign aid. “The health and wellbeing of other countries directly and indirectly affect the health, safety, and economic security of Americans,” the report said. “The United States must preserve and extend its legacy as a global leader, partner, and innovator in global health through forward-looking policies, a long-term vision, country and international partnerships, and most important, continued investment.”
BEWAT Score Is Found to Predict Subclinical Atherosclerosis Without Lab Tests
The Fuster-BEWAT score (FBS)—a simple tool not requiring laboratory tests—predicts the presence and extent of subclinical atherosclerosis with similar accuracy to the standard ideal cardiovascular health score (ICHS), according to a study published in November 2017. The study, in the Journal of the American College of Cardiology, found that the FBS could be a valuable tool in predicting the risk of heart disease and helping patients make lifestyle changes to lower that risk.
BEWAT, which stands for blood pressure, exercise, weight, alimentation (nutrition), and tobacco, was developed by Valentin Fuster, MD, PhD, Director of Mount Sinai Heart and Physician-in-Chief of The Mount Sinai Hospital. The ICHS, which has been recommended by the American Heart Association for use in primary prevention since 2010, includes the same five risk factors in addition to blood tests for glucose and cholesterol. In evaluating the scores’ predictive value, researchers studied 3,983 men and women in the Progression of Early Subclinical Atherosclerosis (PESA) cohort, an ongoing prospective study of employees of Banco Santander in Madrid who are 40 to 54 years old and free of cardiovascular disease.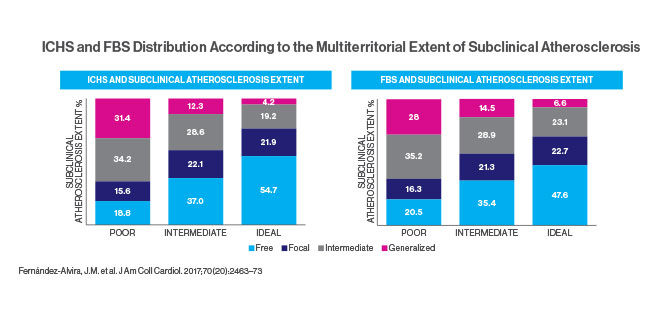
“Looking at these five factors is nearly as accurate as adding on established blood tests to measure cholesterol and blood sugar levels,” says Dr. Fuster, an author of the study conducted at the National Center of Cardiovascular Investigation in Madrid.
Imaging studies of the subjects included 2D vascular ultrasonography of carotid arteries, infrarenal aorta, and iliofemoral arteries and CT scans of coronary arteries. Plaques were defined as any focal protrusion measuring more than 0.5 mm or more than 50 percent thicker than the surrounding intima-media. Coronary artery calcium scores were graded from <1 (mild evidence of coronary artery disease) to ≥400 (extensive evidence). The study compared these imaging results to the subjects’ FBS and ICHS, which classified them as having poor, intermediate, or ideal cardiovascular health. For example, the “ideal” category called for blood pressure below 120/80, body mass index below 25, consumption of more than four servings of fruits and vegetables daily, moderate exercise (150 minutes or more a week) or vigorous (75 minutes or more), and no smoking for the past year. Only 6.5 percent of the subjects earned that score.
The study concluded that the ICHS and FBS showed comparable value in predicting the extent of subclinical atherosclerosis in healthy individuals. However, the FBS is simpler and more affordable because it does not require blood tests. Dr. Fuster says that using the five BEWAT indicators will help doctors, especially those in locations with limited services, to evaluate their patients quickly and easily.
Earlier in 2017, another study by Dr. Fuster’s team found that the experimental 3D vascular ultrasound (3DVUS) could be used to detect atherosclerosis early, inexpensively, and without radiation. In the study, published in March 2017 in the Journal of the American College of Cardiology, researchers explored the bilateral carotid and femoral arteries of 3,860 members of the PESA cohort, measuring global plaque burden. Researchers found that at an average age of 45 years, the plaque burden in subjects was more than twice as high in men as in women (63.4 cubic mm versus 25.7), and higher in the femoral arteries and with increasing age. The 3DVUS examinations were performed using a Philips iU22 ultrasound system equipped with a VL13-5 linear volume array transducer.
The study found that global plaque burden—the sum of all plaque volume in the carotid and femoral territories—had a stronger relationship with cardiovascular risk than the detection of plaque presence alone. Dr. Fuster says further study is needed, but “this novel method is valid for imaging superficial peripheral atherosclerosis burden from early to advanced stages of disease and can be applied to identification of individuals at risk as well as to targeting or monitoring treatment.”
ReChord Trial Is Advancing Minimally Invasive Mitral Valve Repair
Mount Sinai Heart is leading a multicenter clinical trial of a minimally invasive mitral valve prolapse repair technology that has the potential to transform the standard of care for the disorder. The ReChord Trial is a randomized U.S. Food and Drug Administration study exploring the NeoChord DS1000 system in treating patients with degenerative mitral valve regurgitation. Ahmed El-Eshmawi, MD, Assistant Professor of Cardiovascular Surgery, Icahn School of Medicine at Mount Sinai, says the appeal of the device is that it enables surgeons to replace damaged chordae tendineae with artificial chordae without using cardiopulmonary bypass or cutting the sternum.
“While the heart is beating, you make a small incision under the patient’s left nipple, insert the device, and implant the artificial chords to the mitral valve,” says Dr. El-Eshmawi, the site Principal Investigator for the study. “Under normal physiological conditions, you can use transesophageal echocardiography to control the length of these chords to ensure the mitral regurgitation is corrected and the patient is cured.”
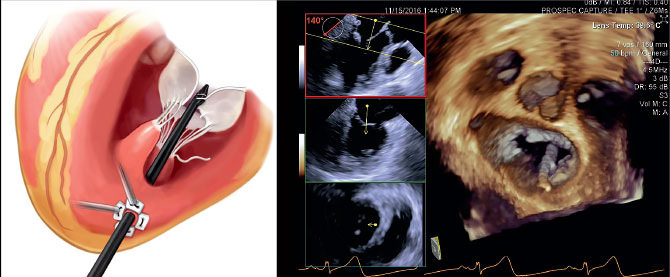
David H. Adams, MD, Cardiac Surgeon-in-Chief of the Mount Sinai Health System, and the Marie-Josee and Henry R. Kravis Professor and Chair of Cardiovascular Surgery, Icahn School of Medicine at Mount Sinai, is National Co-Principal Investigator of the trial. In November 2016, Dr. Adams led a team at The Mount Sinai Hospital that performed the first surgery in the ReChord Trial.
Thirty medical centers and 585 patients with single-segment mitral valve prolapse will participate in the seven-year clinical trial, which is to be completed in July 2023. Dr. El-Eshmawi says half of the subjects will receive a mitral valve repair with the NeoChord device and the control group will receive repair using standard surgical techniques with cardiopulmonary bypass.
Although data on the clinical trial are not yet available, Dr. El-Eshmawi says, “At Mount Sinai, we have noticed that patients in the device group progress sooner following the procedure than those in the surgical control group. In almost all cases, they leave the operating room already extubated. This suggests the technology might lead to a faster recovery rate with a shorter hospital stay than the current standard of care.”
After their procedures, all patients in the trial are monitored through consultations, phone surveys, and echocardiograms to determine the durability of the NeoChord repair compared with the standard of care. Dr. El-Eshmawi says the technology does not use an annuloplasty ring to support the repaired valve, so follow-up is needed to determine the long-term durability. He is optimistic that the NeoChord technology, which has received European Union clearance, could achieve outcomes comparable to standard surgery in well-selected patients.
“I think it will prove to be a safe and effective technology,” he says. “Patients are certainly interested in it as a treatment option. We are getting phone calls from across the country about this trial, so it is gaining popularity very quickly.”
Study Reveals Direct Link Between Surgeon Experience and Outcomes for Mitral Valve Operations
Researchers at the Icahn School of Medicine at Mount Sinai have found that patients who underwent mitral valve operations with surgeons who perform more than 25 such procedures annually experienced lower one-year mortality and reoperation rates when compared to individuals treated by surgeons who do fewer procedures. Significantly, those high-volume surgeons also were more likely to perform a valve repair—the preferred treatment that offers important clinical advantages, such as better life expectancy and quality of life—than a valve replacement with a mechanical or animal valve.
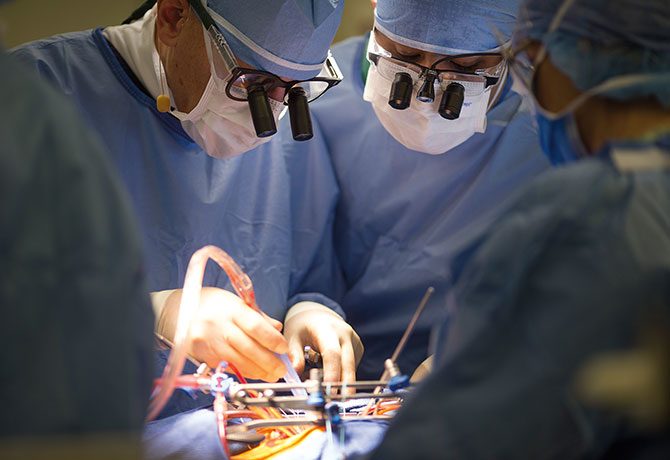
The findings were presented in May 2017 at the American Association for Thoracic Surgery Centennial meeting and simultaneously published online by the Journal of the American College of Cardiology. In a normal heart, mitral valve leaflets open and close with each heartbeat to allow blood to flow in one direction from the upper collection chamber to the lower pumping chamber. In degenerative mitral valve disease, one or both leaflets prolapse, leading to the backward flow of blood (mitral valve regurgitation).
“Our findings add further clarity to the American Heart Association and American College of Cardiology guidelines that already recognize that patients with degenerative mitral valve disease should be referred to experienced mitral surgeons whenever feasible,” says the study’s senior author, David H. Adams, MD, Cardiac Surgeon-in-Chief of the Mount Sinai Health System, and the Marie-Josee and Henry R. Kravis Professor and Chair of Cardiovascular Surgery, Icahn School of Medicine at Mount Sinai. “This is the first study to link individual surgeon volume to survival and freedom from reoperation at one year in patients undergoing operations for degenerative mitral valve disease.”
In the study, Mount Sinai researchers analyzed adult patients who underwent mitral valve surgery in New York State between 2002 and 2013—a population that included 5,475 patients with degenerative disease—comparing repair rates, reoperations within 12 months of repair, and survival, based on total annual surgeon volume. In all, 313 surgeons from 41 institutions met the study’s criteria.
The findings showed that among surgeons who performed any mitral valve procedures, the median volume was 10 cases per year, with a mean valve repair rate of 55 percent. In the subgroup of patients with degenerative disease, the mean repair rate ranged from 77 percent for surgeons with total annual volumes of more than 51 cases to 48 percent for surgeons who performed fewer than 10 cases. Among other key results, surgeons who performed 25 or more surgeries annually had reoperation rates of 1.3 percent compared to 3.6 percent for surgeons who did fewer surgeries. Additionally, survival improved for every 10 additional cases.
The study’s lead author, Joanna Chikwe, MD, Clinical Professor of Cardiovascular Surgery, Icahn School of Medicine, and Professor of Surgery, Chief of Cardiothoracic Surgery, and Co-Director, Heart Institute at Stony Brook University School of Medicine, notes, “Considering that there was an incremental improvement in survival and probability of repair with increasing volume over 25 operations, one could make the argument that a minimum volume target of 50, or even more, annual operations would be optimal and particularly beneficial for surgeons treating patients with complex but repairable mitral valve disease.”
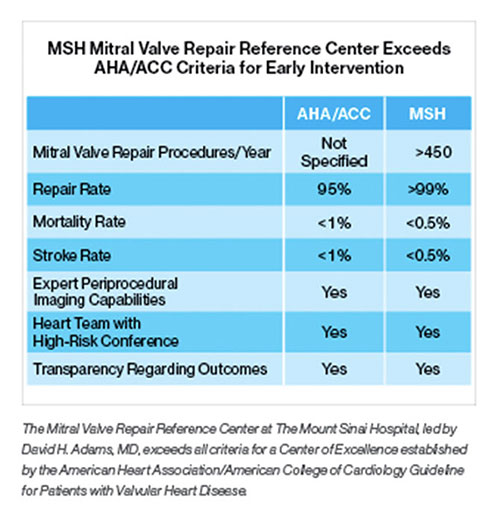
Two Physicians at Mount Sinai’s Cardiac Catheterization Lab Receive Top Safety Rating
For the 19th consecutive year, The Mount Sinai Hospital’s Cardiac Catheterization Laboratory or its interventionalists have received the highest two-star safety rating from the New York State Department of Health (NYSDOH) for percutaneous coronary interventions (PCI), also known as angioplasty.

Mount Sinai’s exceptional ratings appeared in NYSDOH’s May 2017 report on the risk factors associated with PCI at 62 hospitals across the state from December 1, 2011, through November 30, 2014. Two physicians at Mount Sinai Heart, Samin K. Sharma, MD, and Annapoorna S. Kini, MD, were among only three interventional cardiologists in New York State to be awarded a two-star safety rating in two categories for significantly lower mortality rates over a three-year period, during which they performed a combined total of 6,280 cases, according to the report.
“Our long track record of success in offering the highest level of patient safety and excellence in care now spans 19 years,” says Dr. Sharma, Director of Clinical and Interventional Cardiology at The Mount Sinai Hospital, and the Anandi Lal Sharma Professor of Medicine in Cardiology.
During the three-year period, The Mount Sinai Hospital’s risk-adjusted PCI mortality rate for all of its cases—emergency and non-emergency—was 0.75 percent, significantly lower than the statewide average of 1.11 percent and among the top three rates in New York State—while Mount Sinai physicians performed the largest number of PCI procedures (13,029) in the state. For non-emergency cases over that period, Mount Sinai’s PCI mortality rate was 0.44 percent, compared with the statewide average of 0.71 percent, also with the highest volume of procedures.
Dr. Kini, Director of the Cardiac Catheterization Laboratory, and the Zena and Michael A. Wiener Professor of Medicine, says, “The combination of skilled physicians and a team that delivers high-quality patient care, through the use of innovative and evidence-based medical protocols, has contributed to our extraordinary success.”
Bifurcaid App Is a New Teaching Tool for A Challenging Procedure
A new high-tech tool for interventional cardiologists is now available, but not from a medical supplier. It is called Bifurcaid, and it is the brainchild of Annapoorna S. Kini, MD, Director of the Cardiac Catheterization Laboratory. Bifurcaid is a first-of-its-kind app that works on both Apple and Android devices, and is downloadable at no cost via the iTunes or Google Play Stores. Working with the Sinai AppLab, Dr. Kini and her team of fellows released the app in October 2017. Its name refers to coronary bifurcation, one of the most challenging interventional procedures, Dr. Kini says, given the technical expertise and myriad steps required to perform it.
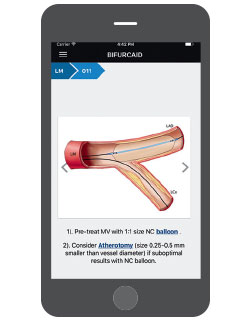
Interventional cardiologists working in cardiac catheterization laboratories have not had an interactive tool to aid them until now. Dr. Kini and her fellows Samit Bhatheja, MD, and Surbhi Chamaria, MD, broke down the process of coronary bifurcation, step-by-step, to make the app user-friendly, working with the AppLab development team, headed by Ashish Atreja, MD, Assistant Professor of Medicine (Gastroenterology), and Pathology, Icahn School of Medicine.
The Bifurcaid app asks users to choose which process they want to explore—left main bifurcation or non-left main bifurcation—and guides them through the procedure. It also includes an overview of bifurcation stenting, with a glossary detailing specific equipment; a section on troubleshooting when wires or stent delivery systems and balloons are difficult to deliver; and hyperlinks to help the user look up critical information without having to exit a step in the algorithm. With the app readily available on the phone, physicians can learn or review whenever they have free time, Dr. Kini says.
In May 2017 Dr. Kini was one of only three interventional cardiologists in New York State to be awarded the prestigious two-star safety rating in two categories by the state Department of Health. “I have developed certain skills in performing these complex cases that I would like to teach other interventionalists so they can perform these challenging cases with ease and have the same excellent results,” she says. “The best way to teach is by writing books, which we have done. But now we are adding new technology into the armamentarium.”
Trial Tests a Therapy for Patients With Ventricular Arrhythmias That Resist Standard Treatment
A clinical trial at Mount Sinai Heart is studying intramural needle ablation (INA) as an option for patients with ventricular arrhythmias that persist after standard radiofrequency ablation.
The Mount Sinai Hospital is one of only three sites in the United States to perform the procedure, says Vivek Y. Reddy, MD, Director of Cardiac Arrhythmia Services, and The Leona M. and Harry B. Helmsley Charitable Trust Professor of Medicine in Cardiac Electrophysiology at the Icahn School of Medicine at Mount Sinai.
The device, made by Biosense Webster, Inc., employs an extendable and retractable needle, introduced through a catheter, to deliver a burst of radiofrequency energy that heats up and destroys tissues causing arrhythmia. While this is similar to the standard procedure, the new needle can reach less accessible sites. The hypothesis of the clinical trial is that INA can end arrhythmias by increasing the density of the current and improving the rate at which transmural lesions are created, with no increased risk of complications.

“This is a promising technology that may allow us to treat arrhythmias that were previously too difficult to ablate due to their origin deep in the tissue, beyond the reach of standard catheters,” Dr. Reddy says.
An early study of eight subjects was published in November 2013 in the journal Circulation by John L. Sapp, MD, and William G. Stevenson, MD, who developed the intramural needle at Brigham and Women’s Hospital in Boston. The study found that INA was feasible and permitted control of some ventricular arrhythmias that had resisted conventional catheter ablation therapy, warranting further study.
Dr. Reddy is Principal Investigator of an expanded 200-subject trial that began in October 2017, seeking results that could lead to approval of the device by the U.S. Food and Drug Administration. The trial will study adult patients with intramural ventricular arrhythmias that could not be terminated by standard ablation. The subjects in the single-arm study will be treated with INA therapy, and the number of recurrent ventricular arrhythmias they experience will be measured six months after treatment. Data collection is to be completed by December 2019.
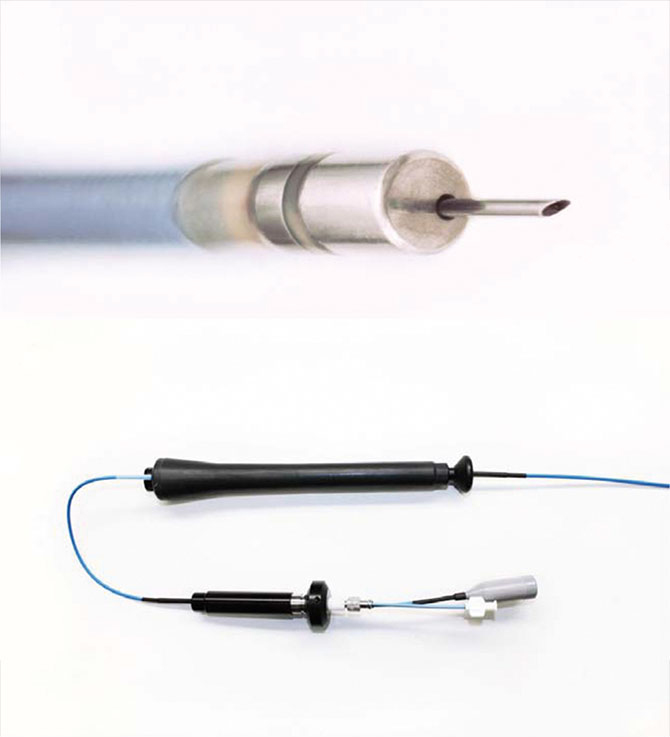
Radiofrequency ablation is the most commonly used method for the catheter treatment of arrhythmias caused by scars from a heart attack or infection that short-circuit the heart’s electrical system. When the source of an arrhythmia is located at a superficial site, the standard catheter is effective at ablation. But the standard approach is less effective at destroying transmural or deep myocardial sites. A number of alternative methods have been attempted to access these sites, such as using 8 mm or 10 mm catheter tips (instead of the standard 4 mm) to deliver radiofrequency energy to a larger area of myocardium. Transcoronary ethanol ablation has also been used with moderate success.
Nevertheless, some sites of arrhythmia cannot be eliminated by standard ablation techniques, often due to deep intramural ventricular tachycardia, which can follow a myocardial infarction. In such cases, the last resort is sometimes open-heart surgery. INA therapy shows promise as a less invasive solution.
Dr. Reddy is a consultant for and receives grant support from Biosense Webster, Inc.
STRIVE Project Traces the “Heart-Brain” Mechanisms Linking Stress and Cardiovascular Risk
An innovative five-year program, led by researchers at the Icahn School of Medicine at Mount Sinai, aims to uncover the mechanisms by which stress contributes to cardiovascular risk and to provide a scientific platform to integrate this knowledge into patient care. It is supported by a $13 million Program Project Grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH).
In the program, called STRIVE (Stress and Trauma: Impact on Cardiovascular Disease), researchers will use noninvasive imaging to study the levels of macrophages—key immune cells contributing to the inflammation that characterizes atherosclerosis—within the arteries, blood, and organs of the immune system.

STRIVE consists of three distinct studies: The first will use mouse models to explore how the brain reacts to stress and affects the immune system to increase cardiovascular disease risk. The second study will translate discoveries from the first to develop imaging methodologies, such as combined MRI and PET scans, to examine these same processes in larger mammals and humans. The third study, for which researchers have recruited the first subjects, will use these imaging technologies to examine the link between increased emotional stress and increased cardiovascular risk in human subjects diagnosed with post-traumatic stress disorder (PTSD).
The project builds on research by Principal Investigator Zahi A. Fayad, PhD, Director of the Translational and Molecular Imaging Institute at the Icahn School of Medicine at Mount Sinai, which for the first time traced the mechanisms that link stress to cardiovascular events, like heart attack or stroke. The findings, published in the January 2017 issue of The Lancet, “provided more evidence of a heart-brain connection,” Dr. Fayad says. “It may seem obvious, but the evidence had not been shown. We had not seen the mechanistic link.”
The Lancet paper was based on two complementary studies. One study was led by the first author of the paper, Ahmed A. Tawakol, MD, Co-Director of the Cardiac MR PET CT Program at Massachusetts General Hospital in Boston. Dr. Tawakol is also a co-investigator in the NIH Program Project Grant. The study analyzed data from 293 people who from 2005 to 2008 underwent PET/CT brain imaging to measure activity in the brain, vascular system, and bone marrow. Researchers found that over the next four years, 22 of the patients had cardiovascular events. In that group, many patients had initially shown a high level of activity in the amygdala, a greater amount of inflammation in the blood vessels, and higher levels of activity in the bone marrow. The latter two factors can contribute to atherosclerosis, which increases the risk for heart disease. This pathway—from emotional stress to increased white blood cell count to inflammation to atherosclerosis—has been identified in animals, but until now, not in humans, Dr. Fayad says.
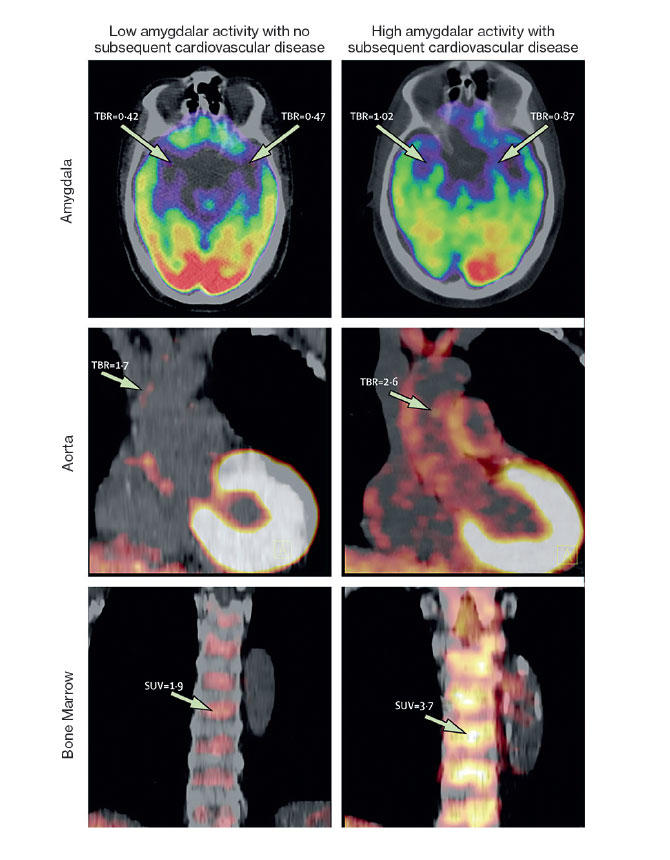
The second study, conducted by Dr. Fayad’s team at the Translational and Molecular Imaging Institute, examined 13 people who were being treated for PTSD at Mount Sinai’s Mood and Anxiety Disorders Program. These patients completed a questionnaire about their perceived stress levels and underwent PET/MR scans. The team found that the patients’ stress levels were linked to increased activity in the amygdala as well as increased inflammation in the blood vessels.
One of the STRIVE studies expands on this research, seeking to study three groups of patients: 80 who are being treated for PTSD; 80 who are “resilient,” with past exposure to trauma but a low perceived level of stress; and 80 who have not been exposed to trauma. The project will explore the possibility that alleviating stress could not only improve patients’ psychological sense of well-being, but also improve their physical atherosclerotic health. “In the future, chronic stress could be treated as a risk factor for cardiovascular disease,” Dr. Fayad says, “so we could screen for it and manage it like other risk factors.”
More information is available at: https://stressppg.com.
Remote Monitoring Improves Readmission Rates for Patients With Congestive Heart Failure
Mount Sinai Heart is reducing readmissions and improving quality of life for congestive heart failure (CHF) patients with remote monitoring using new devices and apps, as well as old-fashioned compassionate care. “We are creating a multimodal way of keeping an eye on our patients after they have left the hospital so that we can optimize their medications and keep them at home—where they want to be—rather than in the hospital,” says Sean P. Pinney, MD, Professor of Medicine (Cardiology), Icahn School of Medicine at Mount Sinai, and Director of Heart Failure and Transplantation, Mount Sinai Health System.

One of the strategies involves the ReDS™ (Remote Dielectric Sensing) system, a wearable vest made by Sensible Medical Innovations. ReDS is based on technology that allows the military to “see through walls” and find survivors in collapsed buildings. In a medical setting, a device sees through the walls of the chest, sending an electromagnetic beam through the middle lobe of the right lung, measuring the lung fluid. Based on the readings, a physician might decide to raise or lower the dosage of diuretics, or hospitalize the patient, if there is an extreme overload of fluid.
Dr. Pinney’s team is participating in a multicenter trial of the device, sponsored by Sensible Medical, that began in September 2015 and is to be completed in June 2018. The trial will compare the readmission rates of 380 patients hospitalized for heart failure. All participants will receive the standard care, including follow-up phone calls and outpatient visits, but one group will also go home with a ReDS vest, with their readings transmitted to care providers. Since July 2017, Mount Sinai has also been using the device in its Rapid Follow-Up Clinic for recently discharged CHF patients. “We are one of only three centers to do this, so we are in the vanguard,” Dr. Pinney says. Among the 28 patients who have used the system since July, the 30-day readmission rate was about 9 percent, compared with 22 percent for heart failure patients overall.
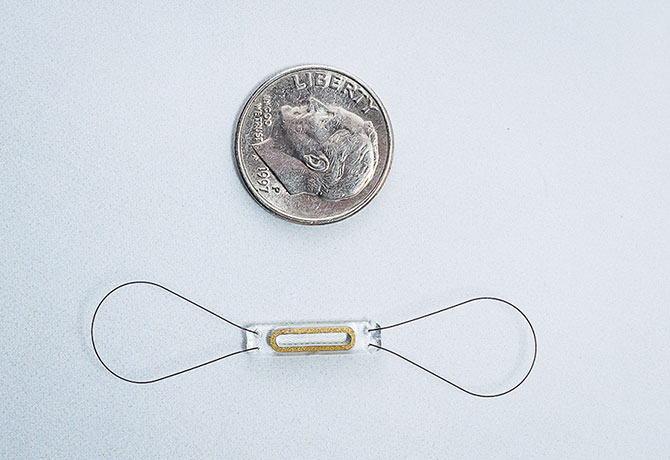
Mount Sinai is an early adopter of another device, CardioMEMS™, an implanted sensor made by Abbott that checks for increased pressure in the pulmonary artery—an early indicator of worsening heart failure. A small pressure sensor is implanted in the distal pulmonary artery using a catheterization procedure. Sensor readings are wirelessly transmitted to a secure website for clinicians.
“If the pressures rise, we increase medication, and if they come down too low, we cut back,” Dr. Pinney says. “So it gives us a feedback loop to get smarter about prescribing medicine. This technology, as part of a management plan, has been shown to keep people out of the hospital, and we are one of only a few dozen centers that use it.”
Mount Sinai is also using apps to help monitor CHF patients. One is HealthPROMISE, a system for iPhone and Android, developed by the Mount Sinai AppLab. Patients are sent home with a blood-pressure cuff and a scale that send data through the app to care providers. “We can track blood pressure, weight, and the answers to simple questions about the patients’ symptoms,” Dr. Pinney says. “Based on that, we can make any adjustments to their prescriptions remotely.” A pilot study by Dr. Pinney’s team found that of 52 subjects using the app, 4 were readmitted within 30 days of discharge. “The CHF patients had a 7 percent readmission rate compared to the national readmission rate of >25 percent within 30 days of discharge,” according to an abstract of the study, presented in October 2017 at the Connected Health Conference in Boston.
Another app, being developed by Dr. Pinney’s group and a startup company, RecoverLINK, is also in clinical trials. It works similarly to HealthPROMISE but asks more detailed questions about patients’ symptoms, mood, compliance with medication, and general quality of life. In addition to remote monitoring, patients also receive personalized video messages from providers.
Dr. Pinney says that heart failure patients often underestimate the severity of their condition, saying “I just have a weak heart,” when the median survival after diagnosis is about five years— “as bad as many cancers, or worse.” He sees a significant opportunity to improve the lives of CHF patients. “There is a need to identify these individuals, refer them to a heart failure Center of Excellence like ours at Mount Sinai, and take advantage of the pharmacologic and device therapies that now exist.”
“Our program is pretty special, in part because the providers here—our nurses, nurse practitioners, and physicians—are very caring and extremely dedicated to their patients,” Dr. Pinney says. “I think it makes a huge difference. If patients feel well cared for, they are more likely to engage with their providers, and that is going to translate into better outcomes for them.”
Gene Therapy That Improved Heart Function In Pigs Is Set for Human Trial
A promising gene therapy that improved heart function in preclinical models of heart failure will proceed to a human trial in 2018, based on research led by Roger J. Hajjar, MD, Director of the Cardiovascular Research Center and the Arthur and Janet C. Ross Professor of Medicine (Cardiology), Icahn School of Medicine at Mount Sinai.
The trial will expand on research, published in October 2017 in the Journal of the American College of Cardiology, that used gene therapy to treat pigs with severe congestive heart failure. In the therapy, an engineered virus was injected into a major artery, delivering a gene meant to regulate protein phosphatase-1. Too much of this protein interferes with the heart’s ability to contract, and Dr. Hajjar says that targeting it could be a way to improve the damaged heart’s pumping action. Dr. Hajjar, a world leader in gene therapy, plans to begin enrolling people with advanced congestive heart failure in a clinical trial in the fall of 2018.

A human clinical trial of a similar therapy in 2015 did not show a significant benefit when it was tested in 250 patients in the United States and Europe. Now the team at Mount Sinai will be using a re-engineered vector that is more effective in delivering to the heart, coupled with a novel gene—a constitutively active form of protein phosphatase inhibitor-1 (I-1c).
The study published in JACC featured two independent experiments on pigs, which have about the same size hearts as humans do, to address the effectiveness, safety, and biodistribution of the gene therapy. The first experiment sought to determine whether the gene-delivery system was safe. The researchers administered a re-engineered vector, BNP116, which was developed from adeno-associated vectors, into 48 healthy pigs. Researchers examined the impact on the functioning of the heart, arrhythmogenicity, blood chemistry, and immune response at different times after the vector delivery.
“No apparent changes in cardiac function were noted, and no events attributable to fatal arrhythmias were documented after vector delivery,” the study notes. In addition, the vector cleared out of the blood within five days after the injection.
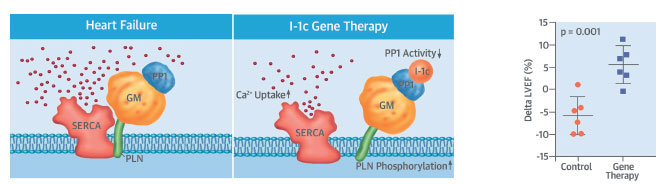
The second experiment looked at the efficacy of the treatment. First, researchers created heart failure in 13 pigs by inducing severe mitral valve regurgitation, and after one month, they measured the pigs’ cardiac performance. Then, six pigs received the gene therapy, and seven pigs received a placebo saline solution.
Two months after the injections, scientists re-examined the heart health of the pigs and found that heart contractility had significantly improved in the pigs treated with gene therapy, with heart failure reduced by 25 percent in the left ventricle and by 20 percent in the left atrium. In addition, the team found a 10 percent reduction of heart size in those pigs. Heart failure in the animals who received the placebo worsened.
“Mount Sinai has performed pioneering work on gene therapy over the last decade, and this study shows that gene therapy is now a viable option for treating patients with advanced heart failure,” Dr. Hajjar said.
Dr. Hajjar is a co-founder of Nanocor, a company focused on the development of gene therapy for the treatment of heart failure.
Study Focuses on Outcomes for Women and Minorities Who Receive PCI With Drug-Eluting Stents
Women and minority-group members who receive percutaneous coronary intervention with drug-eluting stents are at greater risk than white men for major adverse cardiac events such as death, myocardial infarction (MI), or target vessel revascularization (TVR) at 12 months, according to a multicenter study of more than 4,000 patients. The results suggest that both clinical and social factors contribute to the patients’ one-year outcomes.
The study, published online in October 2017 in JAMA Cardiology, analyzed data from the Platinum Diversity Study, a single-arm initiative that involved 1,051 women and minorities at 52 sites across the country. All participants were treated with an everolimus-eluting Promus PREMIER™ stent, made by Boston Scientific Corporation, and researchers collected their socioeconomic data on education, employment, income, and access to health care for further analysis. The Platinum Diversity data were pooled with data from the PROMUS Element™ Plus U.S. Post-Approval Study, which had enrolled an “all-comers” cohort of subjects, also treated with a drug-eluting stent. The pooled analysis covered 4,182 subjects, including 1,863 women (white and minority), 1,059 minorities (men and women), and 1,635 white men. All patients were enrolled beginning in October 2014 and followed for 12 months.

The overall goal of the Platinum Diversity Study is to focus on patients who are underrepresented in clinical trials: women, African Americans, and Hispanics, says Roxana Mehran, MD, Professor of Medicine (Cardiology), and Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, who was Co-Principal Investigator, along with Wayne Batchelor, MD, of Tallahassee Memorial Hospital in Florida. “There is a gap in knowledge and understanding of how to best care for these patients, and I think through this study we are learning a tremendous amount,” says Dr. Mehran, a leading investigator and interventional cardiologist.
The study found that the combined primary endpoint—death, MI, and TVR—at one year was 9.6 percent for minorities, 8.6 percent for women, and 7.6 percent for white men. Data from the individual endpoints revealed a significant difference in the rate of death for white men (2.2 percent) compared with white women (3.4 percent) or minorities (3.7 percent). There was also a higher rate of MI for minorities (3.1 percent) compared with white men (1.1 percent). These risk-adjusted rates took into account baseline clinical, angiographic, and procedural characteristics. Other findings included:
- Higher unadjusted rates of combined death, MI, and TVR for minority women (11.4 percent) compared with white men (7.6 percent)
- Higher unadjusted rates of combined death and MI for minority men (5.9 percent) and minority women (7.6 percent) compared with white men (3 percent).
Women and minorities had a higher adjusted risk of death primarily because of a greater hazard of nonstent-related MI, the study said. Those groups had a higher prevalence of comorbidities, such as diabetes, prior stroke, hypertension, renal disease, and congestive heart failure than white men. But they had lower rates of multivessel disease, prior coronary artery bypass graft surgery, prior MI, and smoking.
The study also examined social determinants of health, identifying a number of independent predictors of adverse cardiac events, including being widowed—as opposed to married or divorced—and lacking private insurance. Income was also a factor: participants who earned less than $25,000 annually had the highest primary composite endpoint of death, MI, and TVR at 12 months (9.8 percent), compared with 4.1 percent for those with annual incomes greater than $50,000. “Given the findings of the study, future trials must focus on the population of women and minorities and evaluate important interventions to enhance adherence, follow-up, and clinical outcomes,” Dr. Mehran says.
Dr. Mehran is a consultant to Boston Scientific.
Message from the Director: Valentin Fuster, MD, PhD
A large clinical trial of a minimally invasive mitral valve repair technique; a promising gene therapy for heart failure; a study of a new tool for catheter ablation; a novel map of the physical link between stress and atherosclerosis—these are some of the latest advances at Mount Sinai Heart. This 2018 issue of the Heart Specialty Report also features an evidence-based plan for the nation’s future role in global health, created by a special committee of the National Academies of Science, Engineering, and Medicine for which I serve as co-chair.

When I established Mount Sinai Heart in 2006, I envisioned a unison of internationally renowned clinical and research experts providing exceptional care for heart disease patients. Today, Mount Sinai Heart is among the world’s leading centers for cardiovascular medicine and advanced diagnostic and therapeutic technologies. For the 19th consecutive year, The Mount Sinai Hospital’s Cardiac Catheterization Laboratory or its interventionalists received the highest two-star safety rating from the New York State Department of Health for percutaneous coronary interventions. And one of our leading interventionalists has developed an app to share her expertise with physicians learning about the procedure.
David H. Adams, MD, Cardiac Surgeon-in-Chief of the Mount Sinai Health System, and the Marie-Josee and Henry R. Kravis Professor and Chair of Cardiovascular Surgery, Icahn School of Medicine at Mount Sinai, found that patients who underwent mitral valve operations with surgeons who perform more than 25 such procedures annually experienced lower one-year mortality and reoperation rates compared to individuals treated by surgeons who do fewer procedures. Our heart failure team used remote monitoring to reduce participants’ readmission rates and improve quality of life. A research group found disparities in outcomes for women and minorities who received PCI with drug-eluting stents. And a study found that the BEWAT score, a set of heart-health indicators that I developed, reliably predicts the presence and extent of subclinical atherosclerosis without laboratory tests.
Through all this growth and accomplishment, as the Icahn School of Medicine at Mount Sinai celebrates its 50th year, we keep in mind that simply listening to patients at the bedside remains medicine’s most indispensable tool.