Coronary Stent Patients May Not Need Long-Term Aspirin
Results from international clinical trial ‘TWILIGHT’ could change standard of care for high-risk cases
High-risk patients undergoing coronary stent procedures may not require long-term aspirin use after stent placement, Mount Sinai researchers report in a groundbreaking study.
The current standard of care for these patients is to combine aspirin with an anti-clotting medication, such as ticagrelor, to lower the risk of heart attack. However, this treatment also increases bleeding complications, and identifying therapies that lower bleeding without increasing heart attack risk has emerged as a clinical priority.
New results from the study, “Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention” (TWILIGHT), suggest that doctors treat high-risk cardiac patients following percutaneous coronary intervention (PCI) by withdrawing aspirin and using ticagrelor alone. The findings were published in the September 26issue of The New England Journal of Medicine and presented as a late-breaking trial at the 31st Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific conference of the Cardiovascular Research Foundation.
“This pivotal, first-ever study to withdraw aspirin in complex patients with coronary stents underlines the importance of bleeding avoidance strategies for targeted care. We showed that withdrawal of aspirin after three months in patients already on a potent antiplatelet regimen (ticagrelor) reduced bleeding significantly without the harm of increasing death or heart attacks,” said TWILIGHT's Global Principal Investigator, Roxana Mehran, MD, Director of the Center for Interventional Cardiovascular Research and Clinical Trials at Mount Sinai Heart and Professor of Cardiology, and Population Health Science and Policy, at Icahn School of Medicine at Mount Sinai. “This simpler approach saved many bleeding events and preserved the benefit of the single potent blood thinner (ticagrelor). We thank our global investigators in 187 sites around the world who worked tirelessly to bring this study to the finish line, and the patients who agreed to participate in this investigator-sponsored study.”
“As an academic, investigator-initiated global trial, this effort represents the concerted and enthusiastic support of partners and collaborators within the United States and abroad who were committed to answering an important and clinically relevant question,” says Usman Baber, MD, MS, Chair of the TWILIGHT Clinical Coordinating Center and Assistant Professor of Medicine at the Icahn School of Medicine at Mount Sinai. “The overall magnitude and consistency of effect we observed with ticagrelor monotherapy suggests this may be a safer and effective antiplatelet strategy as compared with standard of care.”
Current medical guidelines for patients receiving a cardiac stent in a cardiac catheterization laboratory through a minimally invasive PCI procedure advise that patients receive dual-antiplatelet therapy with both aspirin and a drug from a class of stronger antiplatelet medications called P2Y12 inhibitors, of which ticagrelor is one. These medications prevent blood clots that can lead to heart attack or stroke by reducing the ability of platelets, cellular fragments circulating in the blood, to stick to one another and form a clot. TWILIGHT examined the impact of ticagrelor alone versus ticagrelor plus aspirin on clinically relevant bleeding among patients at high ischemic or bleeding risk undergoing PCI.
The investigators enrolled 9,006 high-risk patients at 187 sites across 11 countries in the United States, Canada, Europe, and Asia. All patients had undergone successful PCI with at least one drug-eluting stent and had been discharged on dual antiplatelet therapy with aspirin and ticagrelor for a three-month duration. After completing the three-month course of dual-antiplatelet therapy, patients without major adverse events were then randomized in a double-blind fashion to either aspirin (81 to 100 mg daily) or placebo, and all patients continued with open-label ticagrelor (90 mg twice daily) for 12 months. Investigators tracked the occurrence of any clinically relevant bleeding, or adverse events such as death, heart attack, and stroke.
For patients who took ticagrelor and no aspirin (placebo), bleeding was reduced by 44 percent, compared to patients on ticagrelor combined with aspirin. Additionally, there were no differences in the risk for heart attack, death, or stroke between the groups, which suggests that aspirin withdrawal does not compromise safety. These results were consistent in both men and women, and in patients older and younger than 65 and those with diabetes (patients over 65 and diabetics are often at higher risk of bleeding and ischemic complications after stenting).
“The TWILIGHT trial is a landmark trial which will change our PCI practice by eliminating aspirin after three months in patients on ticagrelor with resultant lower vascular bleeding and no effect on ischemic endpoints,” says Samin K. Sharma, MD, Director of Clinical and Interventional Cardiology for the Mount Sinai Health System. “The findings are particularly important since this is the first study of its kind where a majority of patients were from the United States of America. The results are more generalizable to American patients and the practice of dropping aspirin may be widely and easily accepted in this country.”
“This groundbreaking study enhances Mount Sinai Heart’s excellence in the field of interventional cardiology,” says Valentin Fuster, MD, PhD, Director of Mount Sinai Heart and Physician-in-Chief of The Mount Sinai Hospital. “I am proud of our team for achieving such great success with our collaborators here in the United States and around the world on this pioneering clinical trial.”
The drug ticagrelor is made by AstraZeneca, which provided Mount Sinai with an unrestricted grant to perform the investigator-initiated study. Dr. Mehran has received financial compensation as a consultant and advisory board member for AstraZeneca in the past. Dr. Baber has received consulting fees from AstraZeneca in the past.
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, employing 48,000 people across its hospitals and more than 400 outpatient practices, as well as more than 600 research and clinical labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time—discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 9,000 primary and specialty care physicians and 11 free-standing joint-venture centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida. Hospitals within the System are consistently ranked by Newsweek’s® “The World’s Best Smart Hospitals, Best in State Hospitals, World Best Hospitals and Best Specialty Hospitals” and by U.S. News & World Report's® “Best Hospitals” and “Best Children’s Hospitals.” The Mount Sinai Hospital is on the U.S. News & World Report® “Best Hospitals” Honor Roll for 2024-2025.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Twitter and YouTube.
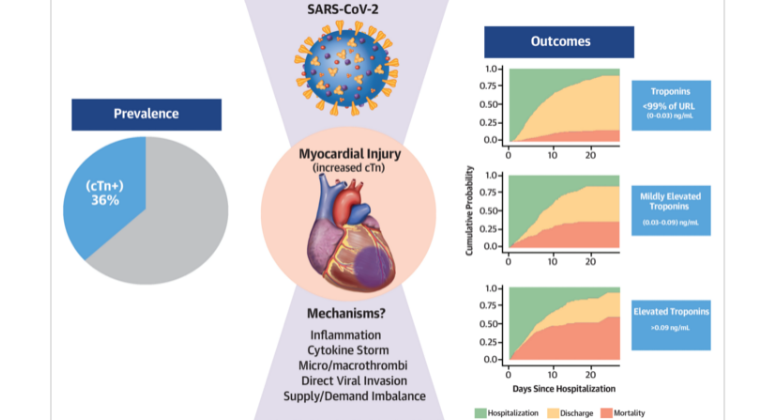
Heart Injury Among Hospitalized COVID-19 Patients Associated with Higher Risk of Death
Jun 08, 2020 View All Press Releases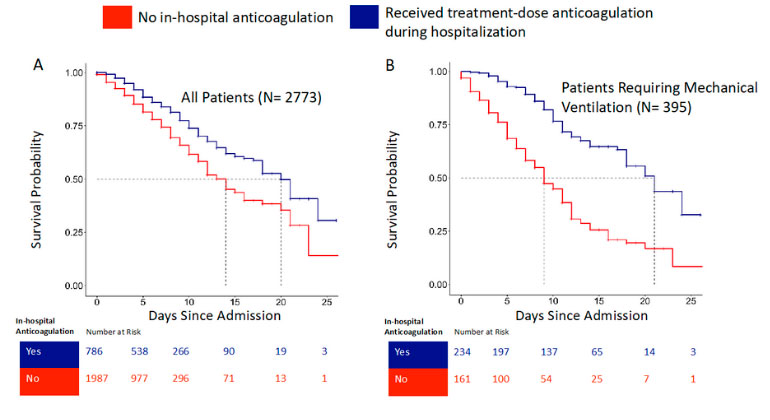
Blood Thinners may Improve Survival among Hospitalized COVID-19 Patients
May 06, 2020 View All Press Releases