Mount Sinai Researchers First to Identify That Two Separate Eye Diseases May Contribute to Common Blinding Eye Condition
Study could lead to early diagnosis and treatment of age-related macular degeneration
Two separate eye diseases may contribute to age-related macular degeneration (AMD), a leading cause of blindness in the United States, according to a new study from New York Eye and Ear Infirmary of Mount Sinai.
The research, published January 9 in Eye is the first to demonstrate that two different types of deposits in the retina may contribute to early AMD, which can progress to advanced AMD and blindness. These two diseases could be diagnosed, studied, and treated separately with appropriate early intervention to prevent vision loss and other complications.
Age-related macular degeneration results from damage to the central area of the retina called the macula, which is responsible for reading and driving vision for driving Nearly 20 million Americans age 40 and older are living with some form of AMD, according to the Centers for Disease Control and Prevention. AMD in its early form is currently considered to be a single disease with cholesterol-containing deposits. These deposits are known as drusen and subretinal drusenoid deposits (SDDs). Early AMD may progress to blindness in two advanced forms, commonly called wet and dry AMD. The advanced dry form is also called geographic atrophy (GA) by eye specialists.
“An amazing fact is that the retina can generate a fluorescent light, similar to that of a light fixture, but a million times dimmer. For the first time, we were able to measure this dim light, called autofluorescence (AF), with ultra-sensitive detectors to study advanced AMD. We found it was consistently twice as bright in the patients with SDDs as those with drusen when they reached advanced AMD, and came from a unique diseased layer,” explains lead author R. Theodore Smith, MD, PhD, Professor of Ophthalmology at the Icahn School of Medicine at Mount Sinai. “Combined with our prior research, this provides conclusive evidence that two different disease processes in AMD are taking place, one with darker fluorescence and drusen, and one with brighter fluorescence and SDDs, and they need to be treated differently.”
Drusen formation can be slowed by appropriate vitamin supplements to prevent vision loss. Currently, there is no known treatment for SDDs, and they pose a greater threat of advanced AMD. However, in a recent previous study, Dr. Smith and a team of Mount Sinai researchers found that patients with SDDs are likely to have heart damage from heart failure and heart attacks, or advanced heart valve disease, or strokes associated with carotid artery disease.
“We think the SDDs result from deficient blood flow to the eye caused by these vascular diseases. We therefore believe that patients with SDDs should be warned they may have life-threatening undetected heart conditions that should be evaluated and treated. Further research needs to be done in women and disadvantaged groups where neglected heart disease is a serious issue. Eye scans for SDDs and routine cholesterol blood tests could address this. Additionally, treating the cardiovascular condition and restoring the eye’s blood supply may also help the SDDs. This work should prompt retinal specialists to look for both drusen and SDDs with optical coherence tomography (OCT), a standard retinal imaging technique, to best counsel patients.
The new research measured the autofluorescence and evaluated OCT scans in 18 patients (32 eyes) with advanced AMD and geographic atrophy (GA). Because GA can happen in multiple regions of the retina, investigators analyzed 52 GA regions overall. They also selected only patients who had OCT scans over the three previous years so they could determine whether the diseased regions started with drusen, SDDs, or both. 18 of these regions originated from drusen, 12 originated from SDDs, and 22 originated from mixtures of drusen and SDDs. The team then measured the brightness of the fluorescent light coming from these regions with a very sensitive light meter. They found it was twice as bright in patients with SDDs when compared to those with drusen. Specifically, the brightness readings averaged 72 in SDD subjects and 36 in drusen subjects, with values in the mixed group falling in between.
“All these numbers translate into one basic fact—there are two different diseases in AMD, one with drusen and one with SDDs,” says Dr. Smith. “The good news for patients and eye specialists is that in the clinic, we will not need advanced AF measurements to know which form of AMD the patient has. As our research showed, the two forms are associated with drusen and SDDs, and those deposits can be identified by standard retinal imaging. It therefore becomes important to diagnose which form of AMD the patient has for treatment and prevention of disease.”
This study was funded by a Regeneron Pharmaceuticals Investigator-Initiated Study, a Research to Prevent Blindness Challenge Grant, the Macula Foundation, a Bayer-Global Ophthalmology Award, and the International Council of Ophthalmology-Alcon Fellowship.
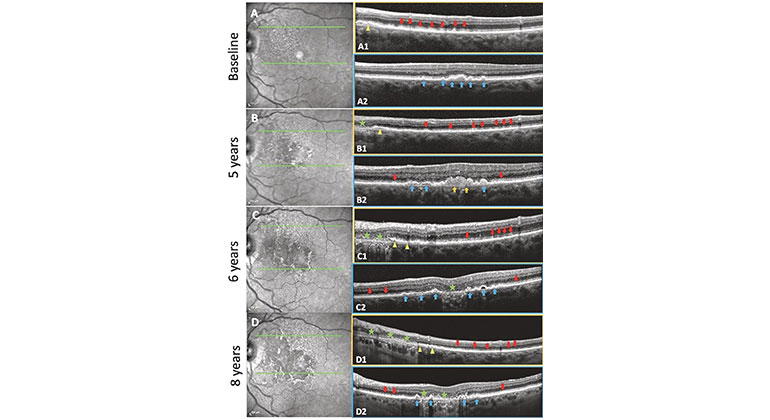
Figure (Credit: Eye): Soft drusen and subretinal drusenoid deposits (SDDs evolving and progressing to geographic atrophy (GA) in the same eye over 8 years with residual basal laminar deposit (BLamD) in a 79-year-old female. Left: NIR images. Right: SD-OCT B scans. Boxes outlined with orange, scans through an SDD region. Boxes outlined with blue, scans through a soft drusen region.
Description: A Baseline. NIR image. Hyporeflectant SDD are largely confined to the supero-nasal macula. Green lines in the NIR image show the horizontal axis and extent of two SD-OCT scans. A1 The scan passes through a region with abundant SDD (red arrows), the next-to-last has broken through the ellipsoid zone, no drusen. Note the splitting of the RPE/BrM complex suggesting BLamD in the nasal aspect of the scan (yellow arrowhead). A2 The scan passes through several large drusen and a pigment epithelial detachment (PED) (blue arrows), no SDD. No atrophy is seen in either scan. B 5-year follow-up. NIR image. SDD have expanded temporally and inferiorly. The SD-OCT scans are placed exactly as at baseline (green lines). B1 There has been some flattening/resorption of former SDD, with new SDD appearing temporally (red arrows). A small area of GA has appeared nasally (green star), with no RPE and a thin line of BLamD, and a split RPE-BL-BrM complex is visible adjacent to the GA area (yellow arrowhead). No drusen. B2 Here, there are adjacent lesions of both types. There has been growth and coalescence of soft drusen (blue arrows) into drusenoid PED (orange arrows). Minimal granular material and Stage 1 SDD have appeared temporally and nasally between the RPE and the ellipsoid zone (red arrows). No atrophy is seen in this scan. C 6-year follow up. NIR image. Confluent SDD can now be appreciated throughout almost all of the macula, including centrally where the NIR reflectance also shows other changes. SD-OCT scans placed as at baseline (green lines). C1 The area of GA has extended temporally with no RPE and scattered BLamD (green stars), adjacent to a region of outer retinal damage and splitting of the RPE/BrM band characteristic of BLamD (yellow arrowheads). SDD temporally are less distinct (red arrows). No drusen. C2 The large central drusenoid PED has collapsed into atrophy (green star), and the other predecessors seen in A2 and B2 have partially collapsed, with remaining soft drusen and PED (blue arrows). Subretinal granular material and Stage 1 SDD still present on either side (red arrows). D 8-year follow-up. NIR image. SDDs are now less apparent superonasally, where atrophy has supervened. SD-OCT scans, same placement (green lines). D1 GA has expanded temporally, with no RPE and a thin line of BLamD (green stars), with further splitting temporally of RPE/BrM band into the SDD area (yellow arrowheads). SDD temporally have regressed almost completely, with preservation of the outer retina (red arrows). No drusen. D2 There is new focal GA nasally and previous GA centrally (green stars) with intervening PEDs (blue arrows).
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, employing 48,000 people across its hospitals and more than 400 outpatient practices, as well as more than 600 research and clinical labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time—discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 9,000 primary and specialty care physicians and 11 free-standing joint-venture centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida. Hospitals within the System are consistently ranked by Newsweek’s® “The World’s Best Smart Hospitals, Best in State Hospitals, World Best Hospitals and Best Specialty Hospitals” and by U.S. News & World Report's® “Best Hospitals” and “Best Children’s Hospitals.” The Mount Sinai Hospital is on the U.S. News & World Report® “Best Hospitals” Honor Roll for 2024-2025.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Twitter and YouTube.